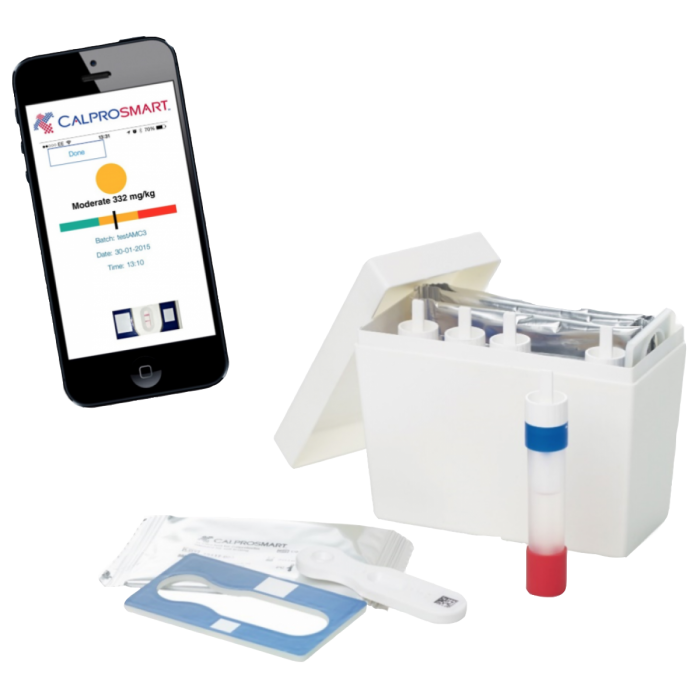
CalproSmart Self-Test Kit
The control in your hands!
Fast and reliable results, in the convenience of your home
The CalproSmart Self-Test Kit is a method for determining calprotectin levels in human stool samples in combination with CalproSmart's specialized application for smartphones. The CalproSmart test allows patients with inflammatory bowel disease (IBD) to monitor their disease level at home, ensuring an effective treatment plan and an early warning in case of relapse. Both the patient and the treating physician are immediately and reliably informed of calprotectin levels. With the CalproSmart Self Test you can monitor the disease... from the convenience of your own!
Χαρακτηριστικά Προϊόντος
- Product code
- CAL201
- Assay target
- Calprotectin
- Package contents
-
- 1 single-use cassette (test)
- 1 toilet seat cover
- 1 pre-filled sample extraction device
- 1 test reference frame (reusable)
- 1 pair of gloves
- 1 instruction sheet in Greek
- Technology
- Lateral flow
- Read-out of results
- Quantitative with color coded presentation
- Range
- 77-1500 mg/kg
- Assay time
- 18 minutes
- Detection system
- Mobile App - CalproSmart App
- Storage
- 2 - 8 °C
- Regulatory status
- IVD, CE labeled
- Official manufacturer website
- https://www.svarlifescience.com/calpro-products/cal230-cal240-cal250
Οδηγίες Χρήσης
Registration to the CalproSmart Application
- You must have been registered at the hospital/clinic by the attending physician. Once registration is complete, you will receive an e-mail to the e-mail address you have provided.
- After setting up the password, you will be able to download the CalproSmart app via App Store or Google Play.
- You should to accept the End User License Agreement (EULA End User License Agreement) before you can use the app.
Test Procedure
- To extract a stool extract (sample) using the stool extract device, rotate the white lid counterclockwise and pull the lid and lever upwards. Insert the end of the rod into the faecal sample so that both slots of the rod are completely filled. If possible, repeatedly insert the rod into different parts of the stool sample. Be careful not to fill the slots with air bubbles. Also avoid seeds, fibres, etc.
- Hold the tube with the blue cap (adapter) and push the stool rod through the opening in the blue adapter. Excess faeces are removed when entering the hopper. Turn the white cap clockwise on the blue adapter until you hear a click and it stops turning. Make sure the tube is completely closed and shake vigorously for 3 minutes.
- Place the rapid examination cassette in the support frame.
- Remove the red cap and discard the first drop. Wipe the end of the device with paper to ensure it is dry.
- Hold the device vertically over the test cassette and add 2 drops (equivalent to 80 µl) to the rapid test cassette. Wait 15 minutes at room temperature to complete the incubation of the sample.
IMPORTANT NOTE: Normally you will see the liquid you added moving in the cartridge window. If this is not observed within 30 seconds, add an additional drop of extract to achieve normal flow.
- Log in to the CalproSmart app. An internet connection is required.
- Hold your smartphone horizontally over the support frame containing the rapid test cassette.
- The image is automatically captured when the barcode, exam bar and control bar are recognized by the app on the smartphone. The intensity of the color of the bars indicates to the app the concentration of calprotectin in the patient's stool in mg/kg.
- The result is displayed in the form of a traffic light, which indicates the patient's disease status
-
- a. green (0-200 mg / kg) = mild disease status
- b. yellow (200-500 mg / kg) = moderate disease status
- c. red (over 500 mg / kg) = severe disease status
- The test results are automatically sent to the CalproSmart portal, which is accessible to your doctor.
IMPORTANT NOTE: It is important to follow the instructions for use carefully in order to achieve an accurate result. Incorrect performance of the procedure, incorrect amount of stool specimen collected, or incorrect volume of extracts added to the rapid test cartridge may result in false positive or negative results.
To download the instruction sheet click here.
To watch a video of the test procedure click here.
Important Information
Please read the enclosed instruction sheet carefully before performing a test.
Diagnosis should not be based on a single test result. It is important that the interpretation of the results be made by the treating physician. Although diagnosis should be based primarily on clinical history and symptoms, CalproSmart test results are a valuable aid in determining further testing such as endoscopy. The user of the CalproSmart self-examination MUST consult the treating physician before adjusting his/her medication based on the results, unless previously agreed upon with the physician.
CONTRAINDICATIONS
- False negative results may occur in patients with granulocytopenia due to bone marrow suppression.
- Patients receiving azathioprine may also have granulocytopenia resulting in false negative results.
- Some patients who are taking non-steroidal anti-inflammatory drugs (NSAIDs) will experience elevated levels of faecal calprotectin.
- The results may not be clinically applicable in children younger than 2 years of age, as these children often have elevated levels of faecal calprotectin.
- Other intestinal diseases, including many gastrointestinal infections and colorectal cancer, may cause elevated levels of calprotectin.
- Faecal calprotectin is a non-specific marker of inflammation in the intestine. The occurrence of elevated levels does not necessarily mean that the patient has active IBD. The complete clinical picture should always be evaluated.
CAUTIONS
According to Article 1(2b) of European Directive 98/79/EC the use of in vitro diagnostic medical devices is intended by the manufacturer to ensure the suitability, appropriate performance and safety of the product. Therefore, the examination procedure, information, precautions and warnings in the instructions for use must be explicitly followed. The use of test kits with analysers and similar equipment must be verified. Any change in design, composition and test procedure and any use in combination with other products not approved by the manufacturer is not permitted. The user shall be solely responsible for such changes. The manufacturer shall not be liable for incorrect results and events due to the above causes. The manufacturer is not responsible for results through visual analysis of patient samples.
- For in vitro diagnostic use only (within test tube).
- Read the instructions for use carefully before performing the test.
- Avoid splashing the liquid or contact with the skin, as skin and eye irritation may occur. If the liquid comes into contact with the eyes, rinse thoroughly with plenty of water. DO NOT ingest. KEEP AWAY FROM CHILDREN!
- Ensure that the extraction device is in a vertical position over the application opening when the 2 drops of extract are added to the rapid test cartridge. If the extraction device is tilted, the drops may have the wrong volume.
- DO NOT read the same examination cassette multiple times. If the time limit for reading the test is exceeded, then the test must be performed from the beginning with a new extractor and a new rapid test cassette.
- Ensure that both slots in the extraction device rod are completely filled. For very watery samples, insert the rod deep into the sample in order to fill the slots completely.
- Do not perform the test in a dark room.
- Do not interchange reagents from different production batches.
- Do not use reagents from other manufacturers together with reagents from this kit.
- Do not use reagents that have exceeded the expiration date indicated on the label
- The extraction solution contains sodium azide at a content of less than 0.1% (by volume).